Formed at the beginning of the COVID-19 pandemic, Operation Warp Speed (OWS) is the Federal Government’s path-breaking mobilization of public and private sector resources to rapidly develop, produce, and distribute multiple safe and effective vaccines to protect lives and restore livelihoods by accelerating the end of the pandemic.
To date, OWS has made great strides in fulfilling this mission by identifying promising vaccines early in their development, standardizing testing protocols, preparing manufacturing capacity, and providing infrastructure, operations, and logistics support for vaccine distribution. The CEA estimated in summer 2020 that OWS could provide an economic benefit of $155 billion if it were to shorten the timeline for vaccine distribution by one month and that the benefit of a vaccine arrival by January 1, 2021 would approach $2.4 trillion. A historical feat, the first vaccines were administered to US citizens 10 days before the end of 2020.
Justification for the OWS effort was based on the understanding that vaccination represented the most critical component to ending the public health crisis and the associated economic damage resulting from pandemic-related lockdowns and private precautionary behavior by people concerned about infection. To ensure rapid mass deployment as soon as clinical trials were successfully completed, OWS prioritized the development of multiple vaccine candidates and the simultaneous enhancement of production capabilities.
OWS procurement and logistics were executed by a combination of Health and Human Services (HHS) and Department of Defense (DOD) acting in close coordination with manufacturers, national pharmacy chains, and national shipping carriers. OWS is distributing the vaccines to 74 jurisdictions made up of US states, territories, and major municipalities as well as federal agencies with direct control over specific populations such as the Indian Health Service, Veterans Health Administration, and the Department of Defense. The overarching objective of OWS is to accelerate the development, manufacturing, and distribution of COVID-19 vaccines, therapeutics, and diagnostics and to produce and deliver 300 million doses of safe and effective vaccines with the initial doses available by January 2021. At this point, there is not a scientific consensus regarding herd immunity. However, one theoretical threshold could be 60% of the population has developed active antibodies to resist the COVID-19 virus.
As of January 11, the US has delivered the first of two doses to 7.7 million individuals with a rate of administered vaccines now approaching 1 million per day. This rate could accelerate to 2 million per day if all jurisdictions operated at the median rate of administration seen to date. At 1 million vaccinations per day and assuming recipients follow through on coming back in for their second dose, herd immunity can be reached by the end of August if the herd immunity threshold is assumed to be 60%. At the 2 million per day rate, this timeline could be moved forward to the end of April.
Keeping up or improving upon such a rapid vaccination rate will require minimizing supply bottlenecks. Compared to other G20 nations, the United States currently has the highest vaccination rate—even higher than that of the UK, which began vaccinating a week earlier. To date, OWS has been highly effective at delivering orders to jurisdictions using FedEx and UPS as the primary logistics carriers. Currently, Pfizer and Moderna will need to continue production as contracted to ensure that enough is available for second doses. However, in the event that all available Pfizer and Moderna vaccines are made available and second doses are not held back, supply should be plentiful to accelerate to 2 million per day.
Across US jurisdictions, some locales are exhibiting very high vaccination rates reaching up to six times the national average. The top five states for vaccination rates are West Virginia, North Dakota, South Dakota, and Nebraska. Figure 1 shows a map of the current performance to date. At this point, no city-specific jurisdiction is outperforming the top ten state jurisdictions. The two best cities for COVID-19 vaccination are the District of Columbia and Philadelphia.
Figure 1 – Percent of Vaccines Administered to Population Aged 18 and above
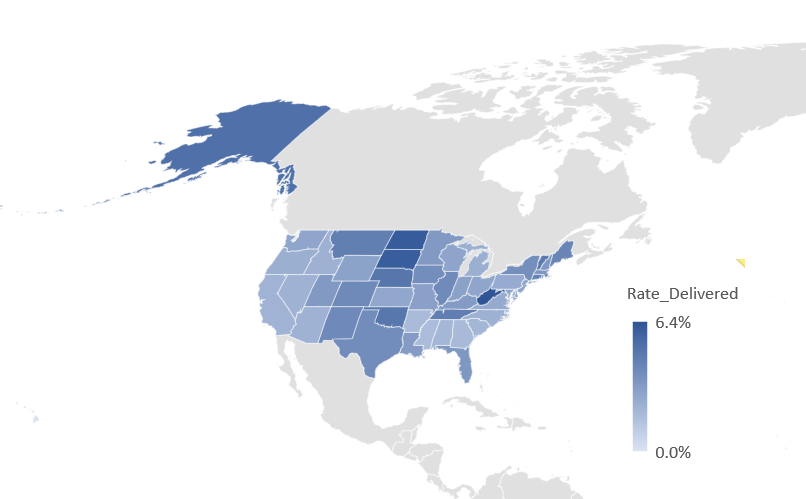
Source: Operation Warp Speed; CEA Calculations
With the United States still in the early stages of mass vaccination, evolving circumstances could merit consideration of future changes to further improve OWS performance. Critically, the effectiveness of OWS depends on timely and accurate reporting. As a result, States should facilitate and encourage immediate day-of vaccine administration reporting. By contrast, some jurisdictions are exhibiting five to six day lags between when a vaccine is administered and when it is reported. More accurate and timely data would help OWS ensure the robustness of supply chains and more seamlessly make logistics and operational decisions to keep the US on the path to herd immunity by summer 2021. This goal will be further aided by the potential arrival of additional vaccine options pending successful Phase 3 clinical trials. At least one of these vaccine candidates has the advantage of requiring only one dose.
Utilization of the National Guard and involvement of the Veterans Administration—both with regard to the veteran and civilian populations—could further aid OWS in achieving rapid, widespread vaccination. These options are not part of the existing OWS strategy, and it is challenging to estimate the marginal benefit of taking this path compared to utilizing the thousands of retail pharmacies available for vaccine distribution that are already under contract to execute widespread vaccination through OWS.
As things stand, OWS has achieved what no previous vaccination effort has even come close to in terms of aiding the development, distribution, and administration of effective vaccines to end a pandemic. If the current pace of vaccination continues to accelerate, and if supply bottlenecks remain at a minimum, it should be feasible for the US to reach herd immunity for COVID-19 by summer 2021, resulting in countless saved lives, restored livelihoods, economic growth, and a return to normalcy.
